
Chemical Change or Physical Change? Sometimes it is difficult to determine whether a change is physical or chemical. Remember that one way often to distinguish a physical reaction from a chemical reaction is that a chemical reaction cannot be easily reversed, if at all. The bubbles are a release of carbon dioxide gas, a product of the chemical reaction between the baking soda and vinegar. When these two household chemicals are mixed together, it immediately starts bubbling and foaming. A common chemical reaction is the mixing of vinegar and baking soda. The creation of a new, solid substance from two liquid substances indicates that a reaction has taken place and altered the original substances. This happens when chemicals dissolved in a solution are mixed together and an insoluble solid, known as a precipitate, forms in the liquid mixture. Another common sign of a chemical reaction is the formation of a precipitate. This is because of chemical reactions that take place as the food begins to break down and go bad, which leads to the formation of new substances that have unique smells associated with them. When food is left out for too long, or it reaches its expiration date, it eventually spoils, often producing a foul odor in its rotten state. Rotting food is an example of odor development as a result of a chemical change. An example of a color change signaling a chemical reaction can be observed when iron reacts with oxygen to produce iron oxide, such as when an iron nail is left outside, and it develops a reddish-brown rust. A chemical cold pack in a first aid kit is an example of a chemical reaction that absorbs heat energy resulting in cooling.
/GettyImages-947148218-1742a84786ae46b0b229da848348fb0f.jpg)
Burning wood is an example of a reaction that releases excess energy as heat. Alternatively, a reaction may require energy from the environment in order to take place, causing heat to be absorbed, and leading to a decrease in temperature. When the chemical bonds of the reactants are broken, sometimes excess energy is released, causing heat to be discharged, and leading to an increase in temperature.
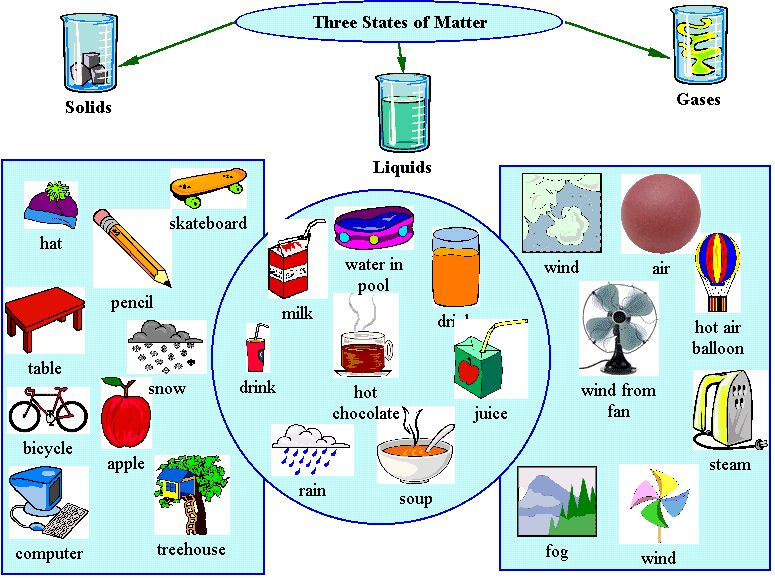
In a chemical alteration, the temperature change occurs as a result of the breaking or formation of chemical bonds. To help determine whether there has been a reaction, chemists consider the basic indicators that a reaction has occurred, such as a change in temperature, a change in color, the development of an odor, the formation of a precipitate, or the formation of a gas. Sometimes it is difficult to tell if a chemical reaction has taken place. This results in the rearranging of atoms in substances to form the products of a chemical reaction, which are brand new molecules that cannot be easily reverted back to their original state. What Is a Chemical Change? A chemical change occurs when the composit ion of a substance is changed, which requires the breaking and forming of chemical bonds during a chemical reaction. The chemical structure of water is the same whether it is a solid (ice), liquid, or gas (steam). Think about ice melting into water, and then water being heated up and turning into steam. Similarly, when a material changes phase, it only changes physically the substance is still the same. A piece of metal may be heated in a fire until it glows, but the metal is the same material before heating and after cooling.

The wood itself has not changed during sanding to become a new material, only the texture of the surface changed. For instance, a block of wood may feel rough when you run your finger across it but rubbing the wood with sandpaper smooths the surface so it no longer feels rough. A change in the texture of a substance is a change in the way it feels. Types of some physical changes are texture, shape, temperature, and a change in the state of matter. What Is a Physical Change? In a physical change, the material involved in the change is structurally the same before and after the change. Chemical changes, on the other hand, are not reversible: A log burned in a fire turns to ashes, but the ashes cannot be changed back into a log. Physical changes in matter are often reversible: An ice cube can melt into liquid water, and then the liquid water can be frozen back into an ice cube. Matter is capable of undergoing changes, which are classified as either physical or chemical.


 0 kommentar(er)
0 kommentar(er)
